The next step in immunotherapy: CAR-T Cells

Our immune system consists of an army of immune cells that all play specific roles in combating foreign bodies. It is their orchestrated response towards pathogens that keep us healthy and functioning. But, what if the enemy comes from within: a group of renegade cells?
Tumor cells were once normal cells, but during replication, their genetic material got mutated, and now they’re growing uncontrollably and causing major collateral damage in their pursuit of proliferation. Due to their nature of being normal cells, our immune system sometimes fails to recognize them as harmful, leading to them growing continuously and causing dangerous cancers.

Figure 1: Cancer cells are usually normal cells that have had their DNA mutated. Image taken from Breast Cancer Prevention Partners.
To give our immune system the boost it desperately needs, scientists have developed a supercharged assassin that may very well tilt the battlefield in our favor.
What are CAR-T Cells
CAR-T cells are the short form of chimeric antigen receptor (CAR) T cells. The chimera, in Greek mythology, is a fire-breathing female monster resembling a lion in the forepart, a goat in the middle, and a dragon behind.
T cells are a subset of immune cells that are specific in their activity and would only kill cells that express a foreign antigen (Sun et al., 2023). However, cancer cells often do not express foreign antigens as they were once normal cells (Kim and Cho, 2022). In order to circumvent this, scientists engineered T cells to express a receptor that redirects T cells to recognize and eliminate cells expressing a specific target surface marker. The reason this can work is because cancer cells tend to upregulate the expression of certain proteins on their surface that can be recognized.

Figure 2: Normal T cell vs CAR-T cell. Normal T cells are also capable of killing cancer cells but are usually less effective due to the low expression of foreign peptides on cancer cells. Image taken from LubioScience.
CAR-T cells got their name as the receptor resembles an assembly of multiple parts from different cell lines. CARs consist of four main components: the antigen-binding domain, the hinge region, the transmembrane domain, and intracellular signaling domains (Ahmad et al., 2022).

Figure 3: On the left is a monoclonal antibody, purple being the variable light chain while light green being the variable heavy chain. On the right is a classic CAR. Image taken from Biology Dictionary.
The antigen-binding domain is the part that the T cells use to detect cancer cells and is derived from the variable heavy and light chains of monoclonal antibodies connected via a flexible linker (Lam et al., 2020).
The hinge region is the extracellular structural region that extends the binding units from the transmembrane domain (Alabanza et al., 2017). The hinge functions to provide flexibility and contributes to the length to allow the antigen-binding domain to access the targeted surface marker (Darowski et al., 2019). The most commonly employed hinge regions are derived from amino acid sequences from CD8, CD28, IgG1, or IgG4 (Sterner and Sterner, 2021).
The transmembrane domain functions to anchor the CAR to the T cell membrane. Most transmembrane domains are derived from natural proteins including CD3ζ, CD4, CD8α, or CD28 (Julamanee et al., 2021). Different transmembrane domains have different advantages as seen by CD3ζ transmembrane domains having more T cell activation while sacrificing stability when compared to CD28 transmembrane domain (Dotti et al., 2014). Furthermore, CAR-T cells with CD8α transmembrane and hinge domains release decreased amounts of TNF and IFNγ and have decreased susceptibility to AICD (a process that decreases T cells count as they get activated) compared to CARs with these domains derived from CD28 (Alabanza et al., 2017).
The intracellular domain is arguably the component that is most highly researched. First-generation CARs contained a CD3ζ or FcRγ signaling domain (Gross, Waks and Eshhar, 1989) but seemed to have low durability, persistence, and therapeutic properties (Till et al., 2008). In response, second-generation CARs with one co-stimulatory domain in series with the CD3ζ intracellular signaling domain were generated, such as the CD28 (Maher et al., 2002) or 4-1BB (Imai et al., 2004) co-stimulatory domains that have since been FDA-approved. It has been hypothesized that co-stimulation through only one domain produces incomplete activation, resulting in the production of third-generation CARs, which incorporate two costimulatory domains in series with CD3ζ, though most of them are still in the preclinical phase (Pulè et al., 2005). CARs incorporating CD28 and 4-1BB signaling resulted in stronger cytokine production in lymphoma, and pulmonary metastasis showed an improved in vivo antitumor response compared to second-generation CARs (Zhong et al., 2010).
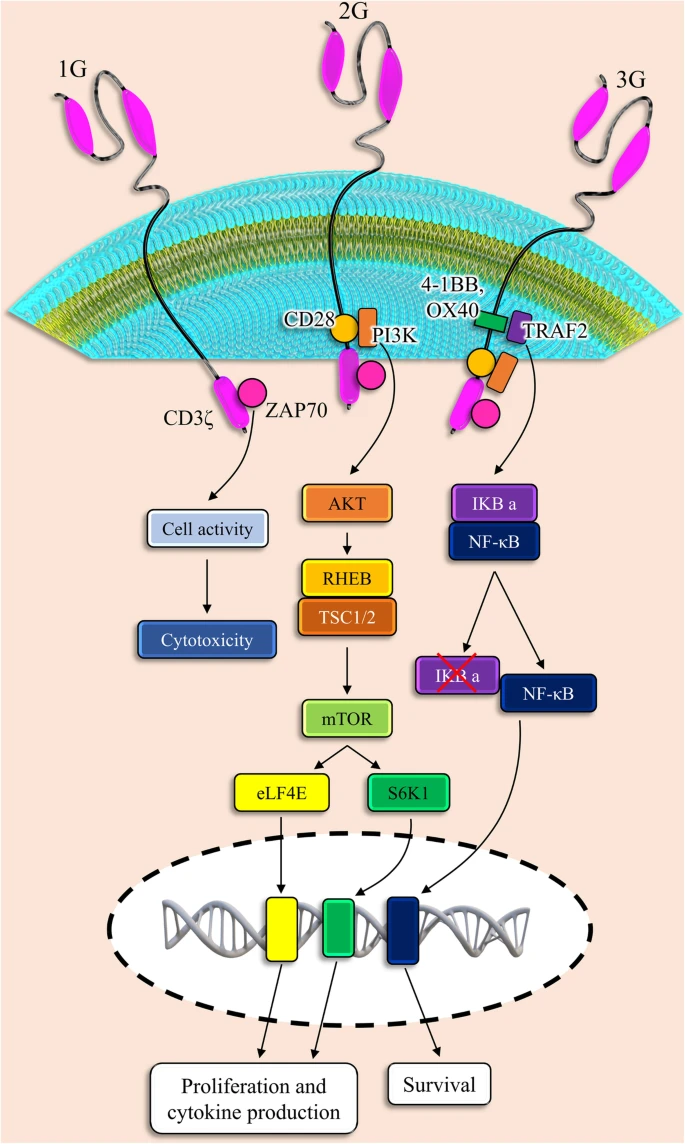
Figure 4: First-generation (1G), second-generation (2G), and third-generation (3G) CARs have different intracellular signaling domains. Image taken from “CAR T cells in solid tumors: challenges and opportunities” (Marofi et al., 2021).
Mechanisms of CAR-T cells
Thus far, six CAR-T cell therapies have been FDA-approved, all for hematological cancer (cancer of the blood). The high response rate achieved with CAR-T treatment for their respective indications was clinically meaningful in those difficult-to-treat patient populations with no other alternative treatment (Srour et al., 2020). To illustrate its efficacy, clinical trials treating patients with second-generation CAR-T cells have generally shown a 60-90% complete remission of their cancer within a month (Chen, Abila and Mostafa Kamel, 2023).
The exact mechanisms for CAR-T cells will differ from formulation to formulation but they follow a general framework. First, T cells are collected from the patient’s blood and then engineered to produce CAR in the lab. The engineered CAR-T cells are reinfused into the patients. The CAR-T cells circulate in the blood and lymph until the CAR finds its target surface marker (antigen) on a cell. The signal is transmitted into its intracellular domain which then triggers the release of perforin and granzymes. These are enzymes that poke holes in the target cell and cause apoptosis, and programmed cell death (Cullen and Martin, 2007). The signal can also trigger growth factors in second-generation and third-generation CAR-T cells to promote persistence and proliferation (Chen, Abila and Mostafa Kamel, 2023).

Figure 5: Killing mechanism of CAR-T cells mediated by perforin and granzyme release. Image taken from “Recent advances and discoveries in the mechanisms and functions of CAR T cells” (Larson and Maus, 2021).
Another mechanism whereby CAR-T cells kill cancer cells is by the Fas receptor and its ligand, FasL. Most importantly, this mechanism works independently of the CAR and can kill antigen-negative cancer cells, with the condition that it has been activated by an antigen-positive cancer cell beforehand (Hong et al., 2018). After activation, CAR-T cells increase their expression of FasL which when bound to the Fas receptor that is highly expressed on cancer cells triggers a cascade of events leading to apoptosis (Nagata and Tanaka, 2017). This can be particularly useful to target a group of heterogeneous cancer cells without creating different CARs for each cancer cell type.

Figure 6: Fas and FasL-mediated apoptosis. Image taken from “Fas–Fas Ligand: Checkpoint of T Cell Functions in Multiple Sclerosis” (Volpe et al., 2016).
Solid tumors: The arch nemesis of CAR-T cells
Although CAR-T cells are exceptionally effective in treating hematological cancer, it is much less capable of fighting solid tumors. This is because solid tumor CAR-T cell therapy is limited by the ability of CAR-T cells to traffic to and infiltrate solid tumors through the vascular endothelium (Salmon et al., 2012). The immunosuppressive tumor microenvironment and physical tumor barriers such as the tumor stroma limit the penetration and mobility of CAR-T cells. In the tumor microenvironment, many cell types that drive immunosuppression can infiltrate solid tumors including myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and regulatory T cells (Quail and Joyce, 2013). In addition, immune checkpoint pathways such as PD-1 or CTLA-4 can serve to decrease antitumor immunity (Sterner and Sterner, 2021). Besides, different levels of antigen expression at various tumor sites may impair the function of CAR T cells at the tumor location because malignant cell antigen diversity makes it difficult to identify tumor cell-specific antigens (Zhang, Gu and Xu, 2018).

Figure 7: Challenges of CAR-T cell therapy in solid tumors. Image taken from “Therapeutic potential of CAR T cell in malignancies: A scoping review” (Mehrabadi et al., 2022).
Of course, scientists are actively devising ways to overcome these obstacles. Utilization of delivery routes other than systemic delivery as local administration eliminates the need for CAR-T cells to traffic to disease sites and limits on-target off-tumor toxicities as the CAR-T cells’ on-target activity is directed on tumor cells minimizing interaction with normal tissues. In fact, preclinical models have demonstrated superior therapeutic efficacy of intraventricular injection of CAR-T cells in breast cancer brain metastases (Priceman et al., 2018) and glioblastoma (Brown et al., 2018).

Figure 8: Advantages of localized CAR-T cell delivery. Image taken from HealthScientific.
CAR-T cells expressing chemokine (molecules that attract immune cells) receptors that match and respond to tumor-derived chemokines also improve CAR-T cell trafficking. For example, recent studies have demonstrated that CAR-T cells modified to express CXCR5 (Whilding et al., 2019) or CAR-T cells overexpressing CXCR1 or CXCR2 (types of chemokine receptors) (Jin et al., 2019) both enhance trafficking and significantly improve antitumor efficacy.

Figure 9: Chemokine receptors help guide CAR-T cells toward the cancer site. Image taken from “CXCR5 guides migration and tumor eradication of anti-EGFR chimeric antigen receptor T cells” (Li et al., 2021).
CAR-T cells also have been engineered to express heparanase, an enzyme that degrades heparan sulfate proteoglycans, a large component of the tumor stroma’s physical barrier. Indeed, they showed enhanced tumor infiltration and antitumor activity (Caruana et al., 2015).
Furthermore, particular CAR-T cells have been engineered to release anti-PD-L1 antibodies to PD-1 (Rupp et al., 2017) and suppress LAG3 (both mechanisms used by cancer cells to evade our immune system) through CRISPR (Zhang et al., 2017). Fourth-generation CAR-T cells, appropriately named TRUCKs, have also been developed that are engineered to release immunostimulatory cytokines such as IL-2 that reduce the CAR-T cell’s vulnerability to the immunosuppressive microenvironment (Chmielewski and Abken, 2020).
Various methods have also been used to overcome the cancer’s antigen diversity, including co-expression of several CARs on a single T cell, programmable CAR expression, possibility of temporary adjustment of target antigens, exploiting of various CAR T cells, the expression of each chimeric receptor relative to a specific antigen, and expression of a chimeric receptor including two or more antigen recognition domains, which in turn leads to the multiple antigens identifying through the individual receptor (Marofi et al., 2021).
Conclusion
Although there are still plenty of limitations of CAR-T cell therapy, there is no denying the capabilities of CAR-T cell therapy, and it doesn’t seem that progress is slowing down either. Let’s all hope that this field continues to flourish and perhaps one day, it’ll be our beacon of hope in eradicating cancer.
Article prepared by: Jared Ong Kang Jie, R&D Director of MBIOS 2023/2024
If you enjoyed this article, do sign up to become a part of our MBIOS family and receive our monthly newsletter along with many more resources in the link below.
References
Ahmad, U., Khan, Z., Ualiyeva, D., Amissah, O.B., Noor, Z., Khan, A., Zaman, N., Khan, M., Khan, A. and Ali, B. (2022). Chimeric antigen receptor T cell structure, its manufacturing, and related toxicities; A comprehensive review. Advances in Cancer Biology - Metastasis, 4, p.100035. doi:https://doi.org/10.1016/j.adcanc.2022.100035.
Alabanza, L., Pegues, M., Geldres, C., Shi, V., Wiltzius, J.J.W., Sievers, S.A., Yang, S. and Kochenderfer, J.N. (2017). Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Molecular Therapy, 25(11), pp.2452–2465. doi:https://doi.org/10.1016/j.ymthe.2017.07.013.
Brown, C.E., Aguilar, B., Starr, R., Yang, X., Chang, W.-C., Weng, L., Chang, B., Sarkissian, A., Brito, A., Sanchez, J.F., Ostberg, J.R., D’Apuzzo, M., Badie, B., Barish, M.E. and Forman, S.J. (2018). Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Molecular Therapy: The Journal of the American Society of Gene Therapy, [online] 26(1), pp.31–44. doi:https://doi.org/10.1016/j.ymthe.2017.10.002.
Caruana, I., Savoldo, B., Hoyos, V., Weber, G., Liu, H., Kim, E.S., Ittmann, M.M., Marchetti, D. and Dotti, G. (2015). Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nature Medicine, [online] 21(5), pp.524–529. doi:https://doi.org/10.1038/nm.3833.
Chen, Y.-J., Abila, B. and Mostafa Kamel, Y. (2023). CAR-T: What Is Next? Cancers, 15(3), p.663. doi:https://doi.org/10.3390/cancers15030663.
Chmielewski, M. and Abken, H. (2020). TRUCKS, the fourth‐generation CAR T cells: Current developments and clinical translation. ADVANCES IN CELL AND GENE THERAPY, 3(3). doi:https://doi.org/10.1002/acg2.84.
Cullen, S.P. and Martin, S.J. (2007). Mechanisms of granule-dependent killing. Cell Death & Differentiation, 15(2), pp.251–262. doi:https://doi.org/10.1038/sj.cdd.4402244.
Darowski, D., Kobold, S., Jost, C. and Klein, C. (2019). Combining the best of two worlds: highly flexible chimeric antigen receptor adaptor molecules (CAR-adaptors) for the recruitment of chimeric antigen receptor T cells. mAbs, 11(4), pp.621–631. doi:https://doi.org/10.1080/19420862.2019.1596511.
Dotti, G., Gottschalk, S., Savoldo, B. and Brenner, M.K. (2014). Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunological reviews, [online] 257(1), pp.107–26. doi:https://doi.org/10.1111/imr.12131.
Gross, G., Waks, T. and Eshhar, Z. (1989). Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences, [online] 86(24), pp.10024–10028. doi:https://doi.org/10.1073/pnas.86.24.10024.
Hong, L.K., Chen, Y., Smith, C.C., Montgomery, S.A., Vincent, B.G., Dotti, G. and Savoldo, B. (2018). CD30-Redirected Chimeric Antigen Receptor T Cells Target CD30+ and CD30− Embryonal Carcinoma via Antigen-Dependent and Fas/FasL Interactions. Cancer Immunology Research, [online] 6(10), pp.1274–1287. doi:https://doi.org/10.1158/2326-6066.CIR-18-0065.
Imai, C., Mihara, K., Andreansky, M., Nicholson, I.C., Pui, C-H., Geiger, T.L. and Campana, D. (2004). Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia, 18(4), pp.676–684. doi:https://doi.org/10.1038/sj.leu.2403302.
Jin, L., Tao, H., Karachi, A., Long, Y., Hou, A.Y., Na, M., Dyson, K.A., Grippin, A.J., Deleyrolle, L.P., Zhang, W., Rajon, D.A., Wang, Q.J., Yang, J.C., Kresak, J.L., Sayour, E.J., Rahman, M., Bova, F.J., Lin, Z., Mitchell, D.A. and Huang, J. (2019). CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nature Communications, 10(1). doi:https://doi.org/10.1038/s41467-019-11869-4.
Julamanee, J., Terakura, S., Umemura, K., Adachi, Y., Miyao, K., Okuno, S., Takagi, E., Sakai, T., Koyama, D., Goto, T., Hanajiri, R., Hudecek, M., Steinberger, P., Leitner, J., Nishida, T., Murata, M. and Kiyoi, H. (2021). Composite CD79A/CD40 co-stimulatory endodomain enhances CD19CAR-T cell proliferation and survival. Molecular Therapy: The Journal of the American Society of Gene Therapy, [online] 29(9), pp.2677–2690. doi:https://doi.org/10.1016/j.ymthe.2021.04.038.
Kim, S.K. and Cho, S.W. (2022). The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Frontiers in Pharmacology, 13. doi:https://doi.org/10.3389/fphar.2022.868695.
Lam, N., Trinklein, N.D., Buelow, B., Patterson, G.H., Ojha, N. and Kochenderfer, J.N. (2020). Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nature Communications, 11(1). doi:https://doi.org/10.1038/s41467-019-14119-9.
Larson, R.C. and Maus, M.V. (2021). Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nature Reviews Cancer, [online] 21(3), pp.145–161. doi:https://doi.org/10.1038/s41568-020-00323-z.
Li, G., Guo, J., Zheng, Y., Ding, W., Han, Z., Qin, L., Mo, W. and Luo, M. (2021). CXCR5 guides migration and tumor eradication of anti-EGFR chimeric antigen receptor T cells. Molecular Therapy Oncology, 22, pp.507–517. doi:https://doi.org/10.1016/j.omto.2021.07.003.
Maher, J., Brentjens, R.J., Gunset, G., Rivière, I. and Sadelain, M. (2002). Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ /CD28 receptor. Nature Biotechnology, 20(1), pp.70–75. doi:https://doi.org/10.1038/nbt0102-70.
Marofi, F., Motavalli, R., Safonov, V.A., Thangavelu, L., Yumashev, A.V., Alexander, M., Shomali, N., Chartrand, M.S., Pathak, Y., Jarahian, M., Izadi, S., Hassanzadeh, A., Shirafkan, N., Tahmasebi, S. and Khiavi, F.M. (2021). CAR T cells in solid tumors: challenges and opportunities. Stem Cell Research & Therapy, 12(1). doi:https://doi.org/10.1186/s13287-020-02128-1.
Mehrabadi, A.Z., Ranjbar, R., Farzanehpour, M., Shahriary, A., Dorostkar, R., Hamidinejad, M.A. and Ghaleh, H.E.G. (2022). Therapeutic potential of CAR T cell in malignancies: A scoping review. Biomedicine & Pharmacotherapy, 146, p.112512. doi:https://doi.org/10.1016/j.biopha.2021.112512.
Nagata, S. and Tanaka, M. (2017). Programmed cell death and the immune system. Nature Reviews Immunology, 17(5), pp.333–340. doi:https://doi.org/10.1038/nri.2016.153.
Priceman, S., Tilakawardane, D., Jeang, B., Aguilar, B., Murad, J., Park, A., Chang, W.-C., Ostberg, J., Neman, J., Jandial, R., Portnow, J., Forman, S. and Brown, C. (2018). Breast Cancer Metastasis to the Brain. Clinical Cancer Research. [online] doi:https://doi.org/10.1158/1078-0432.CCR-17-2041.
Pulè, M.A., Straathof, K.C., Dotti, G., Heslop, H.E., Rooney, C.M. and Brenner, M.K. (2005). A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Molecular therapy : the journal of the American Society of Gene Therapy, [online] 12(5), pp.933–41. doi:https://doi.org/10.1016/j.ymthe.2005.04.016.
Quail, D.F. and Joyce, J.A. (2013). Microenvironmental regulation of tumor progression and metastasis. Nature medicine, [online] 19(11), pp.1423–37. doi:https://doi.org/10.1038/nm.3394.
Rupp, L.J., Schumann, K., Roybal, K.T., Gate, R.E., Ye, C.J., Lim, W.A. and Marson, A. (2017). CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Scientific Reports, 7(1). doi:https://doi.org/10.1038/s41598-017-00462-8.
Salmon, H., Franciszkiewicz, K., Damotte, D., Dieu-Nosjean, M.-C., Validire, P., Trautmann, A., Mami-Chouaib, F. and Donnadieu, E. (2012). Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. Journal of Clinical Investigation, 122(3), pp.899–910. doi:https://doi.org/10.1172/jci45817.
Srour, S.A., Singh, H., McCarty, J., de Groot, E., Huls, H., Rondon, G., Qazilbash, M., Ciurea, S., Bardelli, G., Buck, J., Alousi, A., Nieto, Y., Rezvani, K., Marin, D., Popat, U., Hosing, C., Shpall, E.J., Wierda, W.G., Kantarjian, H. and Champlin, R.E. (2020). Long-term outcomes of Sleeping Beauty–generated CD19-specific CAR T-cell therapy for relapsed-refractory B-cell lymphomas. Blood, 135(11), pp.862–865. doi:https://doi.org/10.1182/blood.2019002920.
Sterner, R.C. and Sterner, R.M. (2021). CAR-T Cell therapy: Current Limitations and Potential Strategies. Blood Cancer Journal, [online] 11(4), pp.1–11. doi:https://doi.org/10.1038/s41408-021-00459-7.
Sun, L., Su, Y., Jiao, A., Wang, X. and Zhang, B. (2023). T cells in health and disease. Signal Transduction and Targeted Therapy, 8(1). doi:https://doi.org/10.1038/s41392-023-01471-y.
Till, B.G., Jensen, M.C., Wang, J., Chen, E.Y., Wood, B.L., Greisman, H.A., Qian, X., James, S.E., Raubitschek, A., Forman, S.J., Gopal, A.K., Pagel, J.M., Lindgren, C.G., Greenberg, P.D., Riddell, S.R. and Press, O.W. (2008). Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood, 112(6), pp.2261–2271. doi:https://doi.org/10.1182/blood-2007-12-128843.
Volpe, E., Sambucci, M., Battistini, L. and Borsellino, G. (2016). Fas–Fas Ligand: Checkpoint of T Cell Functions in Multiple Sclerosis. Frontiers in Immunology, 7(382). doi:https://doi.org/10.3389/fimmu.2016.00382.
Whilding, L.M., Halim, L., Draper, B., Parente-Pereira, A.C., Zabinski, T., Davies, D.M. and Maher, J. (2019). CAR T-Cells Targeting the Integrin αvβ6 and Co-Expressing the Chemokine Receptor CXCR2 Demonstrate Enhanced Homing and Efficacy against Several Solid Malignancies. Cancers, 11(5), p.674. doi:https://doi.org/10.3390/cancers11050674.
Zhang, E., Gu, J. and Xu, H. (2018). Prospects for chimeric antigen receptor-modified T cell therapy for solid tumors. Molecular Cancer, 17(1). doi:https://doi.org/10.1186/s12943-018-0759-3.
Zhang, Y., Zhang, X., Cheng, C., Mu, W., Liu, X., Li, N., Wei, X., Liu, X., Xia, C. and Wang, H. (2017). CRISPR-Cas9 mediated LAG-3 disruption in CAR-T cells. Frontiers of Medicine, [online] 11(4), pp.554–562. doi:https://doi.org/10.1007/s11684-017-0543-6.
Zhong, X.-S., Matsushita, M., Plotkin, J., Riviere, I. and Sadelain, M. (2010). Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Molecular therapy : the journal of the American Society of Gene Therapy, [online] 18(2), pp.413–20. doi:https://doi.org/10.1038/mt.2009.210.
