Monday Article #26: Cancer Drug Resistance
- Admin

- Jul 11, 2022
- 4 min read
Updated: Aug 23, 2022
What is Cancer Drug Resistance?
Chemotherapy has long since been an effective way of treating cancer with some patients achieving remission just by undergoing chemotherapy. However, many patients face the problem where treatment eventually stop working and relapse occur. This phenomenon is known as drug resistance, where cancer cells develop resistance towards the drug used for treatment. Resistance can develop over a matter of weeks, months or years from starting treatment.
Cancer cells can acquire molecular changes due to mutation, ultimately leading to mechanisms that enable them to resist the drug treatment. Such mechanisms involved in drug resistance are reduced drug access to target and quantitative/qualitative changes of drug target.
1. Reduced drug access to target
Cancer cells can reduce drug access to its target by the upregulation of ATP-binding cassette membrane transporter (ABCB1), also known as p-glycoprotein (pgp) or multidrug resistance 1(MDR1). MDR1 is a transmembrane chloride efflux pump that pumps MDR1 substrates such as xenobiotics (foreign compounds) out of the cell before it reaches the cytoplasm (Figure 1). Xenobiotics includes chemotherapeutic drugs such as vincristine, doxorubicin and actinomycin D. Therefore, MDR1 cells can be resistant to many types of drugs hence the name, MDR.
Upregulation of MDR1 in cancer cells leads to increased efflux of drugs and decreased intracellular drug level. For example, when cancer cells are treated with vincristine (a spindle inhibitor), vincristine is pumped out and the intracellular level decreases causing a decrease in the binding and inhibition of tubulin polymerisation to disrupt mitosis thus, unable to induce cell cycle arrest and apoptosis. Cancer cells with upregulated MDR1 are 100 times more resistant to drugs compared to cells with normal level of MDR1.
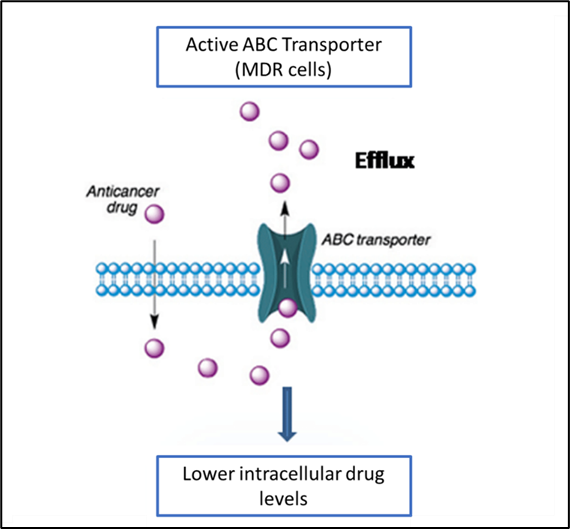
Figure 1 shows the ABC transporter as an efflux pump which pumps anticancer drugs out of cancer cells, causing decrease in intracellular drug level.
2. Quantitative/qualitative changes of drug target
The next drug resistance mechanism is the quantitative or qualitative changes of drug target such as the enzyme dihydrofolate reductase (DHFR) involved in DNA synthesis whereby amplification or mutation of the DHFR gene lead to cancer cells becoming resistant towards methotrexate (MTX), an antimetabolite that competitively binds to and inhibits DHFR activity.
DHFR is involved in the folate cycle (Figure 2) for the formation of thymidine required for DNA synthesis. From figure 2, FH2 is needed to be reduced by DHFR to FH4 for the continuation of the folate cycle. MTX competitively inhibits DHFR, inhibiting the conversion of FH2 back to FH4 thus decreasing the availability of FH4 for the methylation of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). During DNA synthesis, lack of thymidine forces uracil to be incorporated into the DNA. As uracil is toxic towards DNA, this causes DNA double strand break, triggering apoptosis of the cell. MTX has been an efficient anti-cancer therapy since being discovered in the 1940s and is now used to treat a number of cancers including breast cancer, leukemia and osteosarcoma.
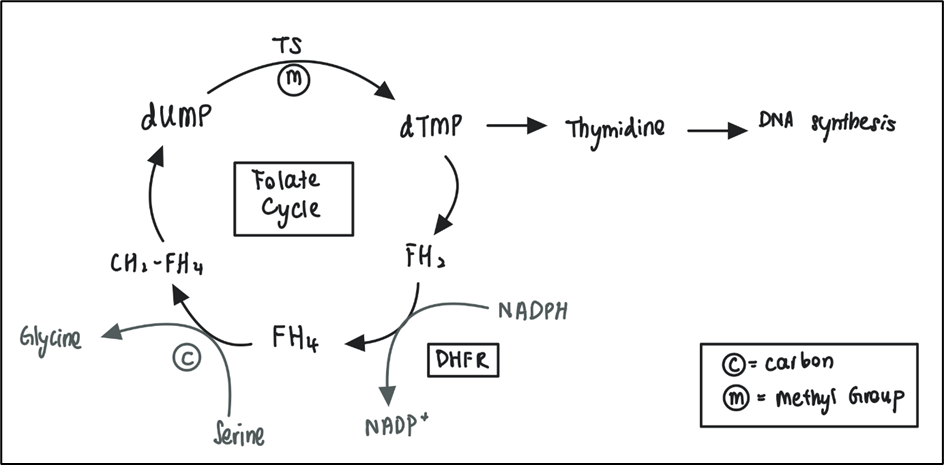
Figure 2 The folate cycle. FH4 receive carbon from serine to convert into CH2-FH4. CH2-FH4 is then oxidised to FH2 by donating a methyl group for the methylation of dUMP to dTMP, catalysed by TS. dTMP is a precursor of thymidine which is used for DNA synthesis. FH2 has to be converted back to FH4 for the continuation of the folate cycle. This step is catalysed by DHFR. (TS= thymidylate synthetase, dUMP= deoxyuridine monophosphate, dTMP= thymidine monophosphate, FH2= dihydrofolate, FH4= tetrahydrofolate, CH2-FH4= methylene tetrahydrofolate, DHFR= dihydrofolate reductase)
However, cancer cells can acquire resistance to MTX by amplification of the DHFR gene, causing MTX to be the limiting factor where there are more DHFR than MTX can inhibit. Mutations in DHFR such as L22R, G15W, and F31W/S can also result in resistance against MTX by decreasing affinity of MTX towards DHFR. These mechanisms cause quantitative and qualitative changes in the drug target (DHFR), enabling cancer cells to become resistant towards MTX.
Ways to lessen the chance of drug resistance occurring
There are several ways to decrease the chances cancer cells acquiring drug resistance such as early detection, de-bulking surgery, combination therapy and using maximum drug dose.
Early detection of cancer enables oncologist to treat cancer patients while cancer cell numbers are still small. Early phase cancer is easier to treat with higher chance of curing the patient and lower mortality rate. Surgery is a standard treatment for cancer patients to remove as much cancer cells as possible before administering chemotherapy. This provides a greater chance of killing most cancer cells and reduce the chances of cancer cells developing drug resistance.
Combination therapy by which administering multiple drugs to target different pathways of killing cancer cells can reduce drug resistance as well as attacking cancer cells from multiple angles, leading to higher chances of cancer cell death. Another related approach is the restrictive combinations where drug dose and administration are given strategically to minimise cytotoxic effect on normal cells but with the same level of toxicity on cancer cell. Using the maximum tolerable drug dose to treat patients can also decrease chances of cancer cells acquiring drug resistance.
Reference:
2. Kantharidis P, El-Osta A, deSilva M, Wall DM, Hu XF, Slater A, et al. Altered methylation of the human MDR1 promoter is associated with acquired multidrug resistance. Clinical Cancer Research. 1997;3(11):2025-32.
3. Wijdeven RH, Pang B, Assaraf YG, Neefjes J. Old drugs, novel ways out: Drug resistance toward cytotoxic chemotherapeutics. Drug Resistance Updates. 2016;28:65-81.
4. Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-43.
This article was prepared by Jennifer Chang

Comments